Your Location:Home > Products > Pharmaceutical intemmediates > 4-tert-Butylthiophenol
CasNo: 2396-68-1
MF: C10H14S
Appearance: clear colorless liquid
4-tert-Butylthiophenol is a thiophenol derivative characterized by a strong odor, a boiling point of 238°C, and a melting point of –11°C. It has a density of 0.99 g/cm³ at 25°C and a refractive index of 1.5485. 4-tert-Butylthiophenol is primarily used in industrial applications due to its reactive thiol group, making it suitable for the production of epoxy resins, phenolic resins, polycarbonate resins, and plasticizers. Additionally, it is used in the manufacture of oil field chemicals, demulsifiers, fragrances, and detergents. Despite its utility, 4-tert-Butylthiophenol poses risks such as serious eye irritation and potential respiratory irritation. Orchid Chemical Supplies Ltd was established in 2009.After more than 15 years of business accumulation, we have established good business relationships with over 25500 major factories in China, can quickly and efficiently connect with high-quality and reliable manufacturers. At the same time, we cooperate with more than 30 domestic R&D laboratories to customize products for customers, improve efficiency, and reduce costs.
InChI:InChI=1/C10H14S/c1-10(2,3)8-4-6-9(11)7-5-8/h4-7,11H,1-3H3/p-1
In the presence of fac-Ir(ppy)3 (Ir-1) as photocatalyst, 4-tert-butylthiophenol (T1) as hydrogen atom transfer (HAT) catalyst and Cs2CO3 as base (Please see the Supplementary Tables 1–5 in Supplementary Information (SI) for more details), the desired arylcarboxylation product 2a was obtained in 66% yield with high selectivity (Entry 1).
An analogous synthesis 12,13 of the thiocoumarine 6 required 4-tert-butylthiophenol (2) as starting material. The preparation of arenethiols by reduction of arenesulfonyl chlorides with …
RhH(PPh3)4 catalyzes reduction of disulf...
thiophenol

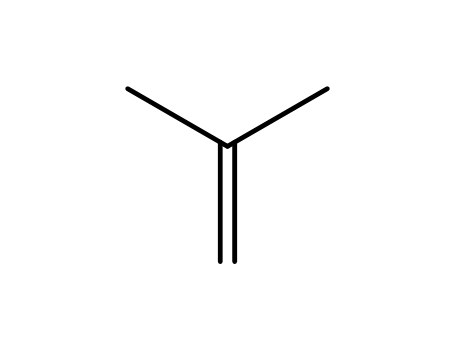
isobutene

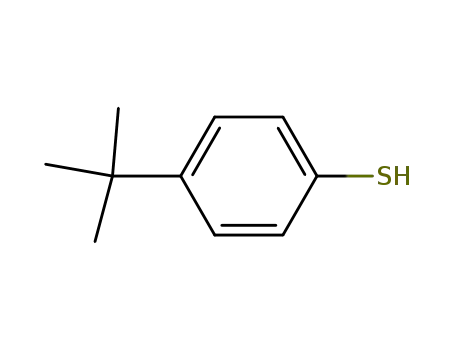
4-t-butylbenzenethiol
| Conditions | Yield |
|---|---|
|
In toluene;
|
68% |
|
With boron trifluoride; at 80 ℃; for 6h;
|
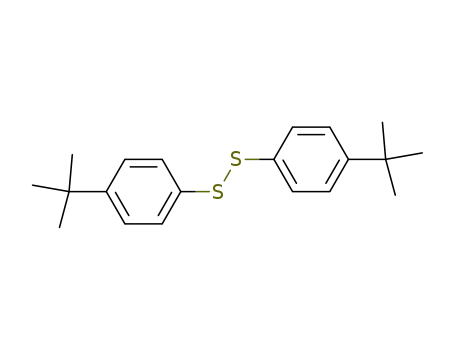
bis(4-tert-butylphenyl)disulfide

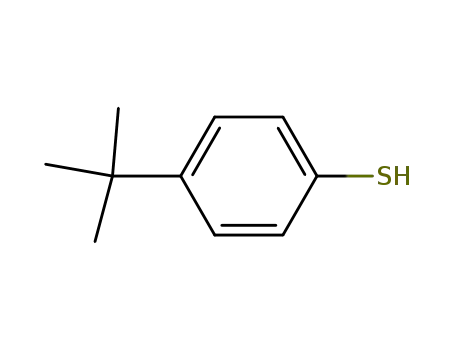
4-t-butylbenzenethiol
| Conditions | Yield |
|---|---|
|
With hydrogen; rhodium hydrido (PEt3)3 complex; In toluene; for 0.5h; Heating;
|
98% |
4-tert-butylbenzenesulfonyl chloride
tert-butyl phenyl sulfide

thiophenol

isobutene
N,N-Bis-[2-(4-tert-butyl-phenylsulfanyl)-ethyl]-propionamide
Thiokohlensaeure-O-p-tolylester-S-<4-tert.-butyl-phenylestser>-imid
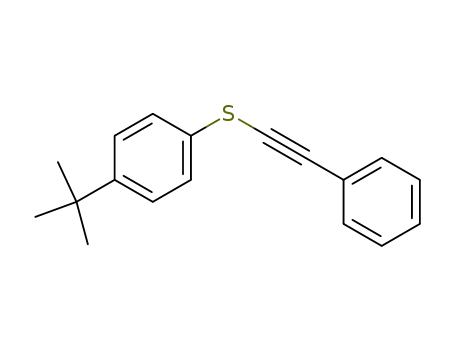
1-Phenyl-2-(p-tert.-butyl-phenyl-mercapto)-ethin
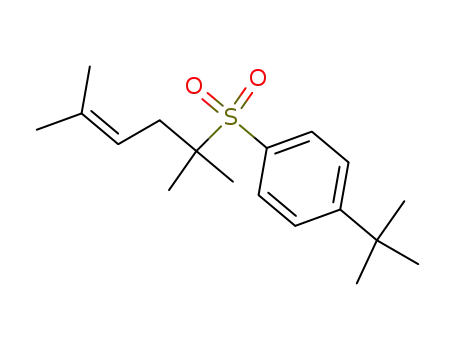
2-<4-tert.-Butyl-phenylsulfonyl>-2,5-dimethyl-hex-4-en