Your Location:Home > Products > Chemical Reagents > 4-Chlororesorcinol
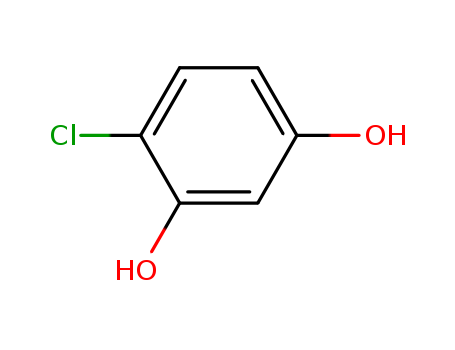


CasNo: 95-88-5
MF: C6H5ClO2
Appearance: White till light pink powder
|
Preparation |
4-Chlororesorcinol is synthesized by reacting resorcinol with dichlorosulfuryl. Resorcinol and diethyl ether were mixed, heated to reflux with stirring, and dichlorosulfuryl was slowly added dropwise. The temperature was then raised to 60°C for 1 h. After recovery of ether, atmospheric distillation is carried out, the distillate is subjected to vacuum distillation again, and the 131°C (0.8-0.93kPa) or 160-164°C (4.0kPa) fractions are collected to obtain the finished 4-chlororesorcinol. |
|
Flammability and Explosibility |
Nonflammable |
|
Safety |
4-chlororesorcinol is classified as a toxic substance and exposure to high concentration of 4-chlororesorcinol could cause several health issues including, skin irritation, serious eye irritation, and other hazards.The safety of 4-Chlororesorcinol has been assessed by the Cosmetic Ingredient Review (CIR) Expert Panel. The CIR Expert Panel evaluated the scientific data and concluded that 4-Chlororesorcinol was safe in hair dye formulations.The CIR Expert Panel concluded that the scientific data suggested that 4-Chlororesorcinol can be safely used in hair dye products. Subchronic dermal exposure to a hair dye product containing 2% 4-Chlororesorcinol produced no evidence of compound-induced toxicity. At the same concentration, no development effects, no evidence of reproductive toxicity, and no carcinogenic effects were seen following dermal exposure. Likewise, 4-Chlororesorcinol was not mutagenic in either a micronucleus or in bacteria, nor did it induce aneuploidy in fungus cells. A 2.5% solution was not a dermal irritant or an ocular irritant. |
|
Purification Methods |
Crystallise it from boiling CCl4 (10g/L, charcoal) and dry it in air. [Beilstein 6 II 818.] IRRITANT. |
|
Application |
In cosmetics and personal care products, 4-Chlororesorcinol is used in the formulation of hair dyes, colors and tints.4-Chlororesorcinol is used in permanent hair coloring systems where color is produced inside the hair fiber. This is accomplished through careful formulation of the product so that the ingredients interact in a highly controlled process. |
InChI:InChI=1/C6H5ClO2/c7-5-2-1-4(8)3-6(5)9/h1-3,8-9H
Synthesis of S-doped activated carbon wi...
A practical and efficient method for the...
Abstract Sonochemical degradation of 4-c...
Candida antarctica lipase B proved to be...
hydrogenchloride

N,N-dichlorourea

recorcinol

4-Chlororesorcinol

4,6-Dichlororesorcinol

2,4,6-trichlororesorcinol

2-chlororesorcinol
| Conditions | Yield |
|---|---|
|
|
N,N-dichlorourea

water

recorcinol

4-Chlororesorcinol

4,6-Dichlororesorcinol

2,4,6-trichlororesorcinol

2-chlororesorcinol
| Conditions | Yield |
|---|---|
|
|
4-chloro-1,3-phenylenediamine
3-chloro-2,6-dihydroxy-benzoic acid
5-chloro-2,4-dihydroxy-benzoic acid
N,N-dichlorourea
6-chloro-7-hydroxy-3,4-dimethyl-2H-chromen-2-one
1,2,4-trihydroxy-9,10-anthraquinone
2',7'-dichlorofluorescein
1-chloro-2,4-bis-trimethylsilanyloxy-benzene