Your Location:Home > Products > Other > Salicylaldehyde
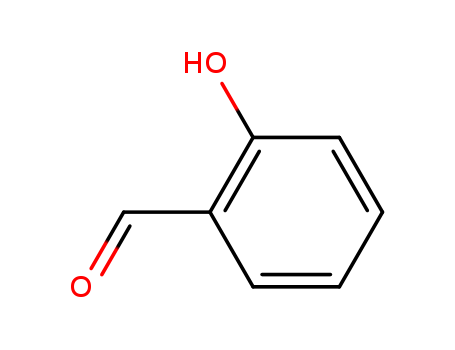


CasNo: 90-02-8
MF: C7H6O2
Appearance: colourless to yellow oily liquid with a bitter almond odour
|
Preparation |
Salicylaldehyde is synthesized from phenol, chloroform, and alkali according to the Reimer–Tiemman method, which was developed in 1876. starting material for the manufacture of coumarin. |
|
Synthesis Reference(s) |
Synthetic Communications, 24, p. 1757, 1994 DOI: 10.1080/00397919408010181 |
|
Reactivity Profile |
Salicylaldehyde is an aldehyde. Aldehydes are frequently involved in self-condensation or polymerization reactions. These reactions are exothermic; they are often catalyzed by acid. Aldehydes are readily oxidized to give carboxylic acids. Flammable and/or toxic gases are generated by the combination of aldehydes with azo, diazo compounds, dithiocarbamates, nitrides, and strong reducing agents. Aldehydes can react with air to give first peroxo acids, and ultimately carboxylic acids. These autoxidation reactions are activated by light, catalyzed by salts of transition metals, and are autocatalytic (catalyzed by the products of the reaction). The addition of stabilizers (antioxidants) to shipments of aldehydes retards autoxidation. |
|
Health Hazard |
Salicylaldehyde is a skin irritant; 500 mg/daycaused moderate irritation to rabbit skin. Itcan have injurious effects on fertility. Studieson rats indicate that subcutaneous administrationof salicylaldehyde in a high doseof >400 mg/kg can produce developmentalabnormalities, fetal death, and postimplantationmortality.The toxicity of this compound, however,is low. No toxic symptoms were noted.LD50 value, oral (rats): 520 mg/kgLD50 value, skin (rats): 600 mg/kg. |
|
Fire Hazard |
Combustible. Can react with oxidizing materials. |
|
Purification Methods |
It is precipitated as the bisulfite addition compound by pouring the aldehyde slowly and with stirring into a 25% solution of NaHSO3 in 30% EtOH, then standing for 30minutes. The precipitate, after filtering at the pump, and washing with EtOH, is decomposed with aqueous 10% NaHCO3, and the aldehyde is extracted into diethyl ether, dried with Na2SO4 or MgSO4, and distilled, under reduced pressure. Alternatively, salicylaldehyde is precipitated as its Cu complex by adding it to warm, saturated aqueous Cu(OAc)2, shaking and standing in ice. The precipitate is filtered off, washed with EtOH, then Et2O, and decomposed with 10% H2SO4; the aldehyde is extracted into Et2O, dried and vacuum distilled. It was also purified by dry column chromatography on Kieselgel G [Nishiya et al. J Am Chem Soc 108 3880 1986]. The acetyl derivative has m 38-39o (from pet ether or EtOH) and b 142o/18mm, 253o/atm. [Beilstein 8 IV 176.] The oxime, [94-67-7] M 137.1, crystallises CHCl3/pet ether (b 40-60o) with m 57o [Beilstein 8 IV 203.] |
|
Waste Disposal |
Salicylaldehyde is burned in a chemicalincinerator equipped with an afterburner andscrubber. |
|
Definition |
ChEBI: Salicylaldehyde is a hydroxybenzaldehyde carrying a hydroxy substituent at position 2. It has a role as a nematicide and a plant metabolite. |
|
Taste threshold values |
Taste characteristics at 20 ppm: spicy, medicinal and astringent |
|
General Description |
Liquid; colorless or pale yellow; bitter almond odor. Sinks and mixes slowly in water. |
InChI:InChI=1/C7H6O2/c8-5-6-3-1-2-4-7(6)9/h1-5,9H
The kinetics of hydrolysis of ortho- or ...
One easy, safe, and effective in situ me...
As the environmental residues of formald...
The intramolecular allylation of carbony...
Among different biological effects of ac...
The rate of hydrolysis of the title imin...
-
Details of the reaction sequence used fo...
The spectroscopic and hydrolytic degrada...
A V2O5@TiO2 catalyzed green and efficien...
The development of non-covalent syntheti...
The present invention relates to process...
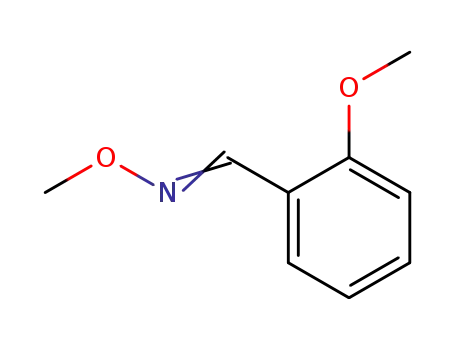
2-methoxybenzaldehyde O-methyloxime

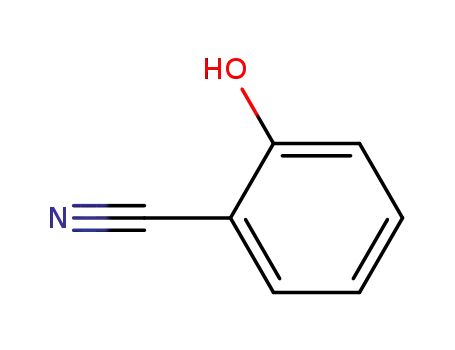
salicylonitrile

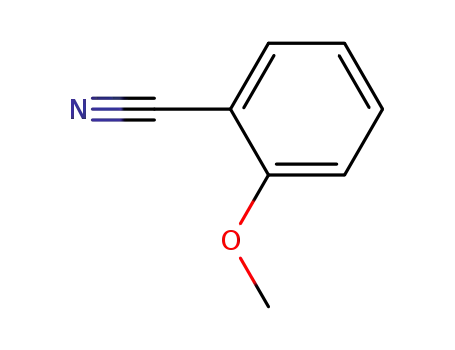
2-methoxy-benzonitrile

salicylaldehyde
| Conditions | Yield |
|---|---|
|
at 650 ℃; under 0.03 Torr;
|
46% 24% 5% |
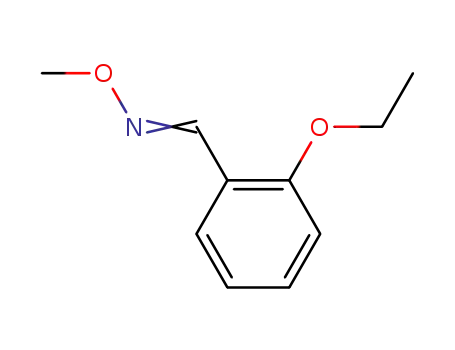
2-ethoxybenzaldehyde O-methyloxime


salicylonitrile

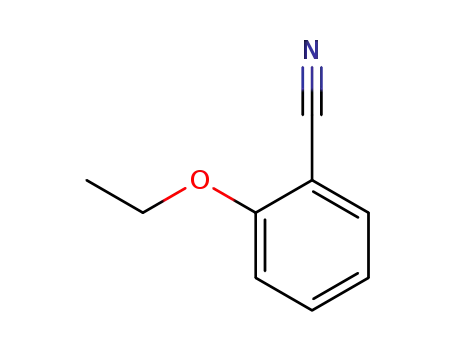
2-ethoxybenzonitrile

salicylaldehyde
| Conditions | Yield |
|---|---|
|
at 650 ℃; under 0.03 Torr;
|
45% 15% 10% |
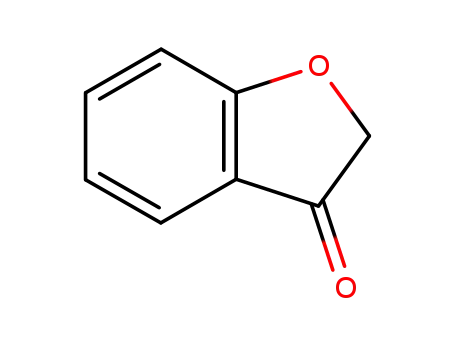
3-oxo-2,3-dihydrobenzo[b]furan
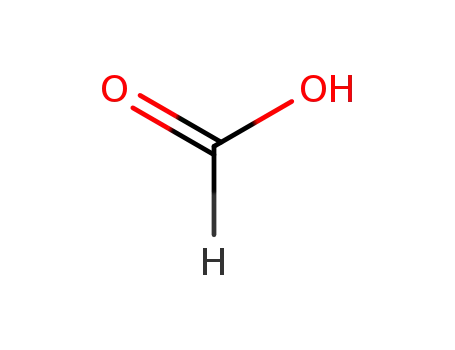
formic acid
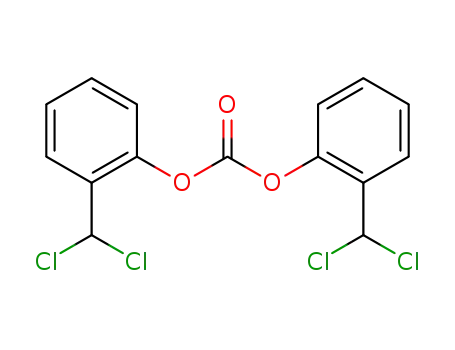
carbonic acid bis-(2-dichloromethyl-phenyl ester)

salicylonitrile
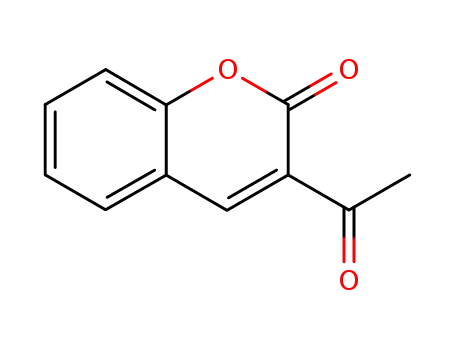
3-acetylcoumarin
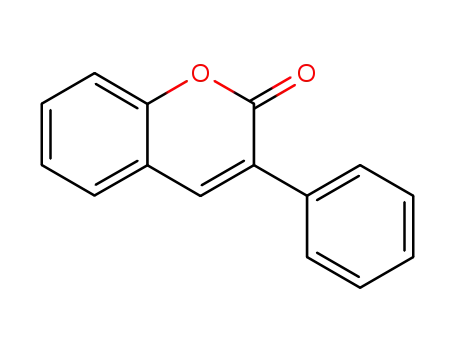
3-phenylcumarin
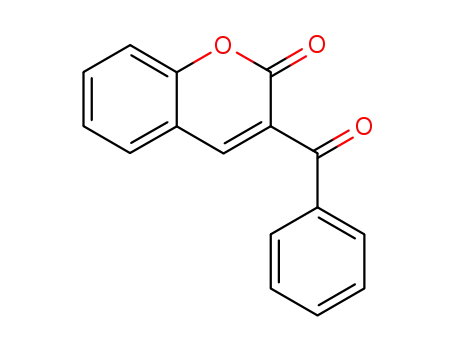
3-benzoyl-chromen-2-one
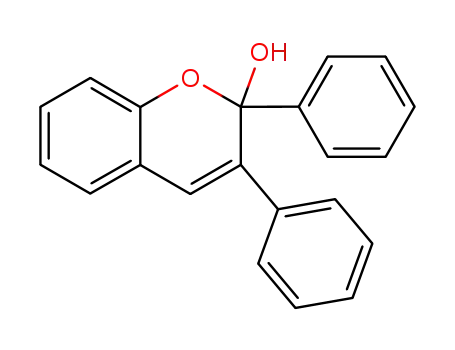
2,3-diphenyl-2H-chromen-2-ol