Your Location:Home > Products > Hair dye intermediates > p-Phenylenediamine
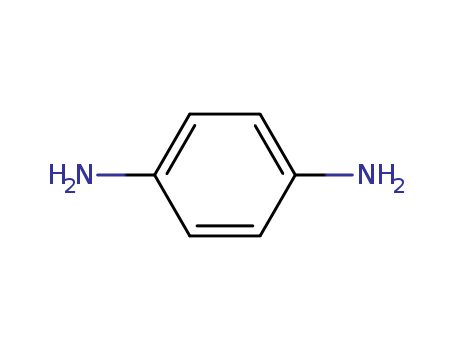


CasNo: 106-50-3
MF: C6H8N2
Appearance: white to light purple solid
|
Production method |
It can be obtained from the reduction of P-nitroaniline via iron in acid medium. Put the iron into hydrochloric acid and heat to 90 °C. Add with stirring of p-nitroaniline. After completion of the adding, have them reacted at 95-100 ℃ for 0.5h, and then add drop wise of concentrated hydrochloric acid so that the reduction reaction is completed. After cooling, use saturated sodium carbonate solution for neutralizing to PH7-8, after boiling and have hot filtration; use host water to wash the filter cake. The filtrate and washings were combined and subject to concentration under reduced pressure; after cooling crystallization or vacuum distillation, we can obtain the p-phenylenediamine with the yield of being 95%. |
|
Acute toxicity |
Oral-rat LD50: 80 mg/kg; oral-mouse LDL0: 100 mg/kg. |
|
Irritation data |
Skin-rabbit 250 mg/24 hours, moderate. |
|
Hazardous characteristics of explosive |
It is explosive when mixed with air. |
|
Flammability and hazard characteristics |
It is combustible upon fire, heat and oxidants with combustion releasing toxic fumes of nitrogen oxides. |
|
Storage characteristics |
Treasury: ventilation, low-temperature and dry; store it separately from oxidants and food additives. |
|
Physical properties |
White, red, or brown crystals. May darken on exposure to air. |
|
Definition |
ChEBI: A phenylenediamine in which the amino functions are at positions 1 and 4 of the benzene nucleus. |
|
General Description |
A white to purple crystalline solid (melting point 234 F) that turns purple to black in air. Flash point 309 F. Toxic by skin absorption, inhalation or ingestion. Used for production of aramid fiber, antioxidants, as a laboratory reagent, in photographic developing, and as a dye for hair and furs. |
|
Air & Water Reactions |
Oxidizes on exposure to air. The finely powdered base if suspended in air poses a significant dust explosion hazard. Soluble in water. Even as a solid will spot downwind areas purple/black (Roger Patrick, DuPont Engineer). |
|
Reactivity Profile |
p-Phenylenediamine is the stongest of the weak aromatic bases. p-Phenylenediamine neutralizes acids in weak exothermic reactions to form salts. May be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Reacts readily with oxidizing agents . |
|
Health Hazard |
p-Phenylenediamine is a moderate to highlytoxic compound; the acute, subacute andchronic toxicity of this amine is greater thanthat of its ortho- and meta-isomers. The acutepoisoning effects in animals were manifestedby lacrimation, salivation, ataxia, tremor,lowering of body temperature, increasedpulse rate, and respiratory depression. Anintraperitoneal dose of 10.8 mg/kg (sus pended in propylene glycol) in male ratscaused the formation of methemoglobin tothe extent of 12.9% after 5 hours (Watan abe et al. 1976). The hydrochloride of thisamine has been reported to cause edemaof the head and neck in animals dosedwith 120–350 mg/kg. p-Phenylenediamine in hair dye formulations produced skinirritation and mild conjunctivial inflamma tion in a variety of test animals (Lloydet al. 1977). In guinea pigs, contact pro duced skin sensitization. Hair dyes con taining p-phenylenediamine damaged visionwhen applied into eyes. In addition, allergicasthma and inflammation of the respiratorytract resulted from exposure to higher con centrations. Reports in early literature citeseveral cases of human poisoning resultingfrom the use of hair dyes containingp-phenylenediamine. The toxic symptomsreported were liver and spleen enlargement,vertigo, gastritis, jaundice, atrophy of liver,allergic asthma, dermatitis, cornea ulcer,burning and redness in eyes, and presbyopia(the latter effects arising from using hair dyeson the eyebrows and eye lashes).Tests for mutagenicity in Salmonellamicrosome assays (in vitro) were negative.With metabolic activation, upon oxidationwith hydrogen peroxide, most mutagenictests showed positive results. Tests forcarcinogenicity were negative, although itslightly increased the overall tumor ratein experimental animals. After oxidationwith hydrogen peroxide, the amine producedtumors in the mammary glands of female rats(Rojanapo et al. 1986). |
|
Fire Hazard |
Combustible material: may burn but does not ignite readily. When heated, vapors may form explosive mixtures with air: indoors, outdoors and sewers explosion hazards. Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated. Runoff may pollute waterways. Substance may be transported in a molten form. |
|
Flammability and Explosibility |
Nonflammable |
|
Biochem/physiol Actions |
p-Phenylenediamine causes allergic reactions with skin. It is widely used in hair dyes. It eventually forms Bandrowski′s base, which is found to be the primary cause for allergy. p-Phenylenediamine exposure results in dermatitis, urticaria and anaphylaxis. It acts as an electron donor and is known to reduce cytochrome c. |
|
Contact allergens |
PPD is a colorless compound oxidized by hydrogen peroxide in the presence of ammonia. It is then polymerized to a color by a coupling agent. Although a wellknown allergen in hair dyes, PPD can be found as a cause of contact dermatitis in chin rest stains or in milk testers. It is also a marker of group sensitivity to para amino compounds such as benzocaine, some azo dyes, and some previous antibacterial sulphonamides. |
|
Safety Profile |
Suspected carcinogen with experimental tumorigenic data. Poison by ingestion, subcutaneous, intravenous, and intraperitoneal routes. Mildly toxic by skin contact. A human skin irritant. Mutation data reported. Implicated in aplastic anemia, Can cause fatal liver damage. The p-form is more toxic and a stronger irritant than the 0and misomers. Wen used as a hair dye it caused vertigo, anemia, gastritis, exfoliative dermatitis, and death. Has caused asthma and other respiratory symptoms in the fur-dyeing industry. Combustible when exposed to heat or flame; can react vigorously with oxidizing materials. To fight fire, use water, Con, dry chemical. When heated to decomposition it emits acrid smoke and irritating fumes. See also other phenylenediamine entries and AMINES |
|
Potential Exposure |
p-Phenylenediamine has been used in dyestuff manufacture, in hair dyes; in photographic developers; in synthetic fibers; polyurethanes, and as a monomer and in the manufacture of improved tire cords. Also used as a gasoline additive and in making antioxidants. |
|
Carcinogenicity |
A number of dermal carcinogenesis bioassays have been reported using p-PDA alone in an organic solvent or in combination with hydrogen peroxide. An 85-week study in which female Swiss mice were treated with 5% or 10% p-PDAin acetone, 0.02 mL/animal applied topically, showed no evidence of carcinogenicity. p-PDA was not found to be carcinogenic when administered by diet to male and female F344 rats and B6C3F1 mice at the dietary doses of 625 or 1250 ppm; the high dose approximated the maximum tolerated dose. An IARC Working Group concluded that on the basis of lack of human data, and inadequate animal data, p-PDA was not classifiable as to its carcinogenicity to humans. A recent meta-analysis of 11 case-control studies and one cohort study of the relationship between p-PDA exposure through use of personal hair dye and bladder cancer did not indicate any causal association. |
|
Source |
Bulk quantitities may contain m- and o-phenylenediamine and aniline as impurities. |
|
Environmental fate |
Biological. In activated sludge, 3.8% mineralized to carbon dioxide after 5 d (Freitag et al., 1985). In activated sludge inoculum, following a 20-d adaptation period, 80.0% COD removal was achieved (Pitter, 1976). Photolytic. A carbon dioxide yield of 53.7% was achieved when phenylenediamine (presumably an isomeric mixture) adsorbed on silica gel was irradiated with light (λ >290 nm) for 17 h (Freitag et al., 1985). Chemical/Physical. p-Phenylenediamine will not hydrolyze because it does not contain a hydrolyzable functional group (Kollig, 1993). |
|
Shipping |
UN1673 Phenylenediamines (o-, m-, p-), Hazard Class: 6.1; Labels: 6.1-Poisonous materials. |
|
Purification Methods |
Crystallise the diamine from EtOH or *benzene, and sublime it in vacuo; protect it from light. The acetate has m 304o. [Beilstein 13 IV 104.] |
|
Incompatibilities |
Dust may form explosive mixture with air. A strong reducing agent. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides, acid chlorides; acid anhydrides; chloroformates, and strong bases. Incompatible with organic anhydrides; isocyanates, aldehydes. Heat and light contribute to instability. Keep away from metals. |
|
Waste Disposal |
Controlled incineration whereby oxides of nitrogen are removed from the effluent gas by scrubber, catalytic or thermal device. |
InChI:InChI=1/C6H8N2/c7-5-1-2-6(8)4-3-5/h1-4H,7-8H2
-
-
-
P, Se-codoped g-C3N4 (PSeCN) nanosheet w...
A simple and inexpensive method for the ...
(Chemical Equation Presented) (Me3Si)3Si...
-
Based on the experimental and DFT calcul...
The one-electron electro-oxidation of 1,...
Photocatalytic hydrogenation of 4-nitroa...
Supported bimetallic Fe–Cu/SiO2 material...
Ni/mZSM-5 and Ni/H-mZSM-5 were synthesiz...
A variety of nitroarenes such as simple,...
Magnetic Fe3O4 nanoparticles (MNPs) were...
A new class of core–shell magnetic gold ...
Selective reduction of nitroaromatic pol...
-
An effective, mild, and clean method for...
The current environment-friendly regulat...
The one-electron oxidation of selected p...
We have demonstrated that a combination ...
-
Single-atom catalysts are emerging as a ...
It is shown experimentally that the hydr...
The purely aqueous-phase reduction of a ...
In this study, a facile method for the s...
Abstract: Nitro-aromatic pollution in in...
Nanoporous silver was used as the cataly...
An alkaliphilic strain Bacillus lentus B...
The kinetics of the liquid-phase hydroge...
Removal of toxic nitroarenes, which thre...
Palladium nanoparticles with unique cata...
Electrochemical amination of aromatic co...
An experimentally easy to perform method...
Flowerlike Bi2S3 microspheres have been ...
-
The electrochemical e.s.r. of 1,4-diamin...
Creatine as the nitrogen-rich, green and...
Seedless, surfactantless and support-fre...
In this study, hyperbranched polyamidoam...
The binuclear ruthenium(II) p-cymene com...
An efficient, mild and selective synthes...
Metal nanoparticles decoration on magnet...
Abstract: An analysis is made of the kin...
Abstract: In this work, we have prepared...
Candida parapsilosisATCC 7330 supported ...
Amines are among the most fundamental mo...
The formation of arene C-N bonds directl...
By utilizing hydrazine (N2H4) as the nit...
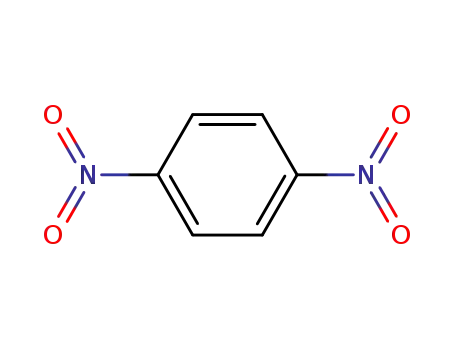
para-dinitrobenzene

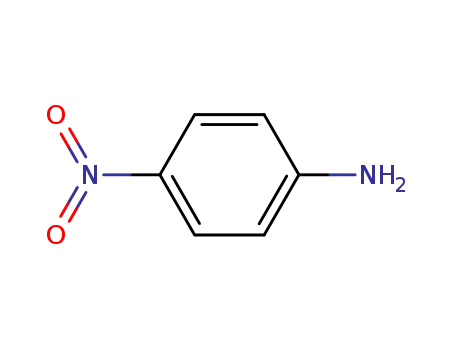
4-nitro-aniline

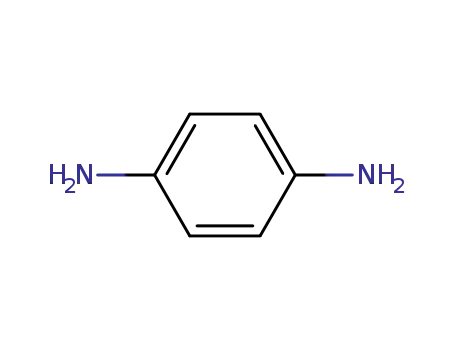
1,4-phenylenediamine
| Conditions | Yield |
|---|---|
|
With tris(triphenylphosphine)ruthenium(II) chloride; formic acid; triethylamine; palladium on activated charcoal; In tetrahydrofuran; at 20 - 25 ℃; for 0.5h; Product distribution; selective reduction with a new hydrogen source;
|
85% 3 % Chromat. |
|
With cerium(IV) oxide; hydrazine hydrate; tin(IV) oxide; In methanol; for 4h; Heating;
|
85% 5% |
|
With hydrogen; trans-Pdpy2Br2; In ethanol; at 30 ℃; for 8h;
|
15% 75% |
|
para-dinitrobenzene; With sodium tetrahydroborate; In water; at 20 ℃; for 0.25h; Inert atmosphere; Green chemistry;
In water; at 20 ℃; for 1.16667h; Inert atmosphere; Green chemistry;
|
74% 21% |
|
With potassium fluoride; polymethylhydrosiloxane; palladium diacetate; In tetrahydrofuran; water; at 20 ℃; for 0.5h;
|
72% 20% |
|
With potassium fluoride; polymethylhydrosiloxane; palladium diacetate; In tetrahydrofuran; at 20 ℃; for 0.5h;
|
72% 20% |
|
With hydrogen; trans-Pdpy2Cl2; In ethanol; at 30 ℃; for 4h;
|
70% 25% |
|
With hydrogen; trans-Pdpy2Cl2; In ethanol; at 30 ℃; for 4h; Product distribution; Mechanism; further catalyst, further reaction time; induction period of the reduction;
|
70% 25% |
|
With sodium tetrahydroborate; water; at 0 - 20 ℃; for 24h;
|
53% 21% |
|
With gum acacia supported platinum; hydrogen; In water; at 20 ℃; under 760.051 Torr;
|
|
|
With titanium dioxide; In ethanol; for 2h; Irradiation; Green chemistry;
|
60 %Chromat. 30 %Chromat. |
|
With titanium(IV) oxide; In water; isopropyl alcohol; for 4h; UV-irradiation; Inert atmosphere;
|
82 %Chromat. 17 %Chromat. |
|
With cadmium sulphide; In isopropyl alcohol; for 2h; Inert atmosphere; Sonication; Irradiation;
|
84 %Chromat. 4 %Chromat. |
|
With cadmium sulphide; In isopropyl alcohol; for 24h; Inert atmosphere; Sonication; Irradiation;
|
72 %Chromat. 11 %Chromat. |
|
With sodium tetrahydroborate; In ethanol; water; at 20 ℃; for 1h; Green chemistry;
|
13 %Chromat. |
|
para-dinitrobenzene; With hydrazine hydrate; In methanol; for 0.333333h; Sonication;
In methanol; at 25 ℃; for 2.8h; Irradiation;
|
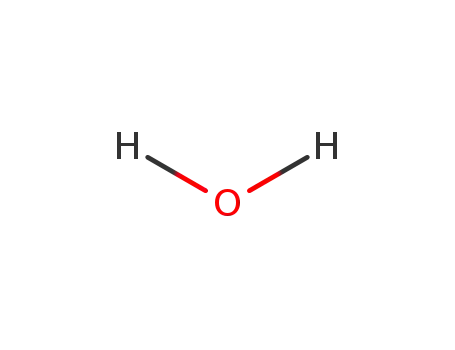
water

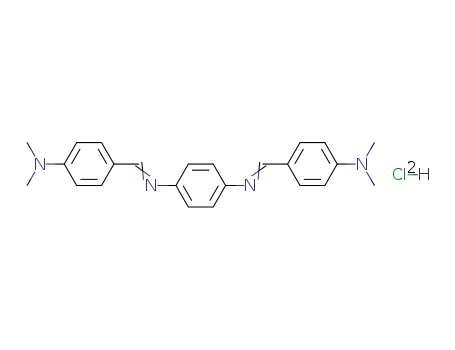
bis-(4-dimethylamino-benzylidene)-p-phenylenediamine; dihydrochloride

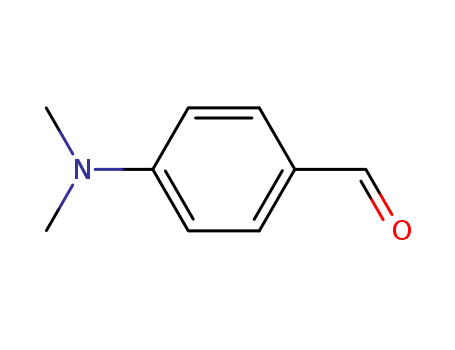
4-dimethylamino-benzaldehyde


1,4-phenylenediamine
| Conditions | Yield |
|---|---|
|
|
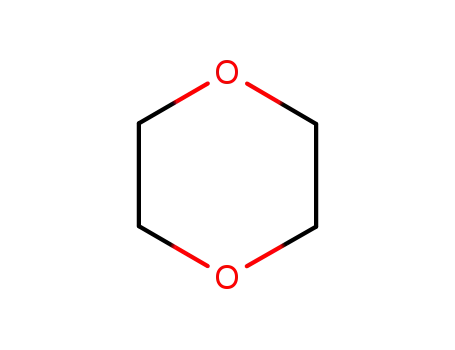
1,4-dioxane
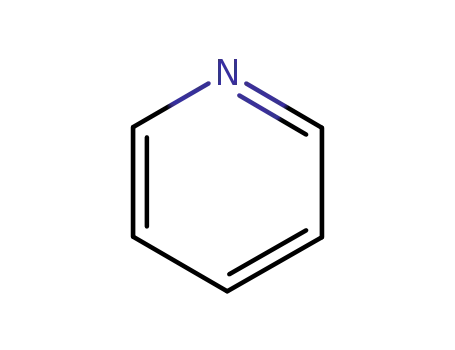
pyridine

para-dinitrobenzene
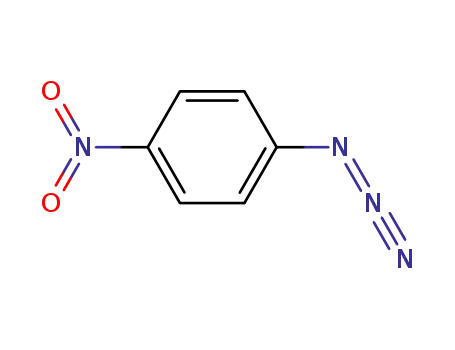
4-nitrophenyl azide
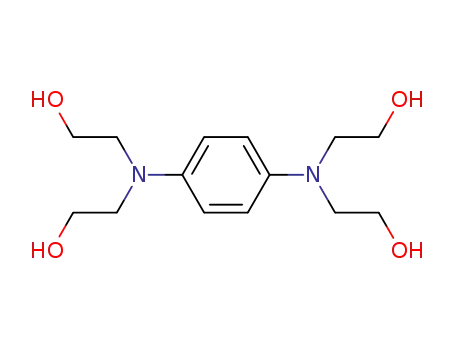
tetrakis-N-(2-hydroxy-ethyl)-p-phenylenediamine
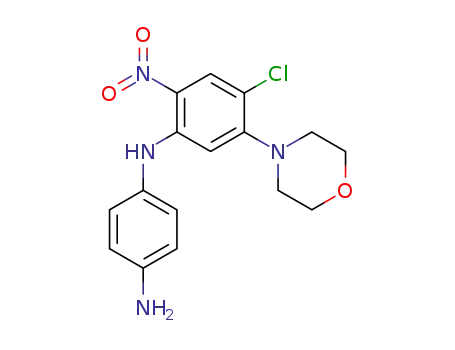
N-(4-chloro-5-morpholino-2-nitro-phenyl)-p-phenylenediamine
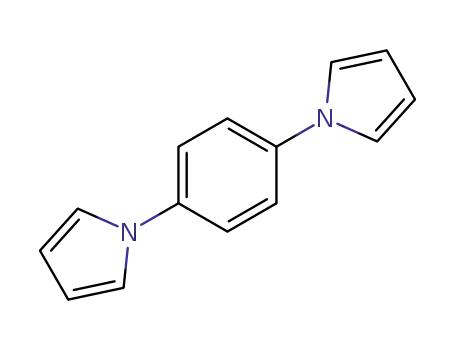
1,4-di(1H-pyrrol-1-yl)benzene
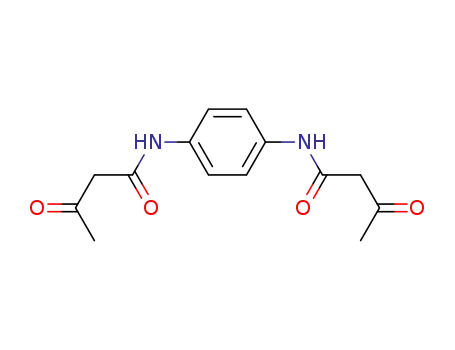
1,4-bis-(acetoacetylamino)-benzene