Your Location:Home > Products > API > L-Glutamine
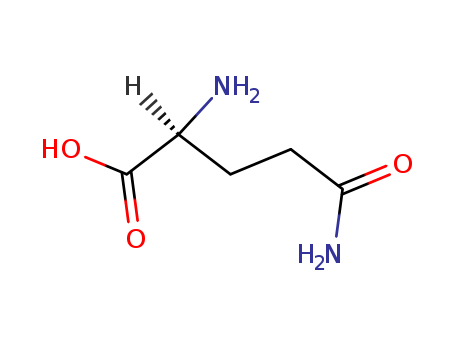


CasNo: 56-85-9
MF: C5H10N2O3
Appearance: White crystalline powder
|
Abundance |
Glutamine is the most abundant amino acid in the body, comprising 60% of the total pool of free amino acids. |
|
Synthesis Sources |
The main synthesis sources of glutamine circulating in plasma are skeletal muscle, adipose tissue, and lungs. |
|
Nitrogen Transport |
Glutamine performs most of the transport of nitrogen from skeletal muscle to visceral tissues. |
|
Primary Fuel |
Glutamine is used as a glucose-efficient primary fuel for rapidly dividing cells, including enterocytes, colonocytes, lymphocytes, and fibroblasts. |
|
Acid-Base Balance |
Glutamine plays a role in acid-base balance through ammonia production in the kidney. |
|
Biosynthesis Substrate |
Its oxidized form provides the substrate for the synthesis of purines and pyrimidines necessary for DNA, RNA, and messenger RNA. |
|
Precursor to Glutathione |
Glutamine is a precursor to glutathione, a powerful endogenously produced antioxidant. |
|
Research Importance |
Glutamine is one of the most researched amino acids in medical nutritional care, including conditions such as gastrointestinal diseases, oncology, burn injury, HIV/AIDS, and chronic wound management. |
|
Role in Cell Metabolism |
Glutamine supplies carbon and nitrogen to fuel biosynthesis, participating in tricarboxylic acid (TCA) cycle supplementation and the biosynthesis of nucleotides, glutathione (GSH), and other nonessential amino acids. |
|
Glutamine Deprivation |
Glutamine deprivation suppresses cancer growth and induces cell death in several cancers due to its metabolic dependency, termed glutamine addiction. |
|
Glutaminase Inhibition |
Specific genetic interference with glutaminase inhibits tumor cell growth, and CB-839, the first glutaminase inhibitor, has entered several clinical trials. |
|
Mitochondrial Glutamine Transporter |
The mitochondrial glutamine transporter, encoded by a transcript variant of the SLC1A5 gene, was only recently discovered. |
|
Plasma Membrane Glutamine Transporters |
Glutamine is transported into cells through plasma membrane glutamine transporters such as SLC1A5, SLC38A1, and SLC38A217, contributing to biosynthesis in the cytoplasm. |
|
General Description |
L-Glutamine is an amino acid and a key building block of proteins in the body. It is classified as a conditionally essential amino acid, as the body can typically synthesize enough of it under normal conditions, but may need additional supplementation during times of illness, stress, or intense physical activity. L-Glutamine plays a crucial role in various physiological functions, including supporting immune system function, maintaining the integrity of the intestinal lining, and facilitating the removal of ammonia from the body. It is also utilized as a source of energy for cells, particularly in the gut and immune system. Additionally, L-Glutamine has been studied for its potential benefits in supporting muscle recovery and reducing muscle soreness after exercise. As a result, it is commonly used as a supplement by athletes and individuals looking to support their overall health and wellness. |
InChI:InChI=1/2C5H10N2O3/c2*6-3(5(9)10)1-2-4(7)8/h2*3H,1-2,6H2,(H2,7,8)(H,9,10)
The present invention relates to a proce...
Amino acids are key synthetic building b...
D-glutamine is a D type stereoisomer of ...
Two new cyclic lipopeptides termed laxap...
(S)-4-Carbamoyl-2-[2-(4-methoxy-phenyl)-2-oxo-ethoxycarbonylamino]-butyric acid

L-glutamine

1-(4-methoxyphenyl)ethanone
| Conditions | Yield |
|---|---|
|
Irradiation; mild conditions;
|
C48H63N9O13

L-valine

L-threonine

L-glutamine

L-phenylalanine

N-methyl-L-tyrosine

L-proline
| Conditions | Yield |
|---|---|
|
With hydrogenchloride; In water; at 110 ℃; for 16h;
|
6-oxo-1,4,5,6-tetrahydropyridazine-3-carboxylic acid
L-glutamine
theanyl-L-alanine (H-Tea-L-Ala-OH)
ammonia
Nα-benzyloxycarbonyl-glutamine
nicotinamide
N-benzoylglutamine
glycine