Your Location:Home > Products > Other > Ethyl acetoacetate
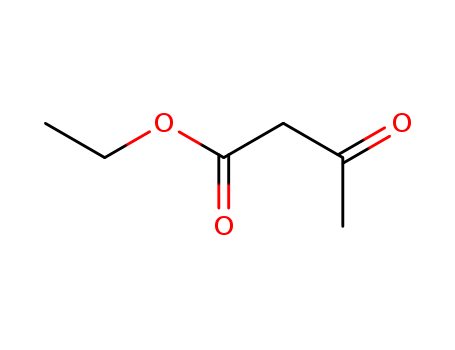


CasNo: 141-97-9
MF: C6H10O3
Appearance: Colourless liquid
Ethyl acetoacetate is an ethyl ester derived from the condensation of acetoacetic acid with ethanol. It is a beta-keto ester that exists in equilibrium between its keto and enol tautomers, with the keto form being predominant. thyl acetoacetate is primarily produced by reacting diketene with ethanol in the presence of an acid catalyst. Orchid Chemical Supplies Ltd was established in 2009.After more than 15 years of business accumulation, we have established good business relationships with over 25500 major factories in China, can quickly and efficiently connect with high-quality and reliable manufacturers.
Uses:
Flavouring Agent: Employed to impart fruity flavors in various food products.
Antibacterial Agent: Utilized in formulations for its antibacterial properties.
Plant Metabolite: Acts as a plant metabolite with various biological functions.
Organic Synthesis: Serves as an intermediate in the production of derivatives of succinic acid and other organic compounds.
Materials Science: Used in the preparation of functionalized materials, such as chitosan and alginate composites for metal ion sorption.
InChI:InChI=1/C6H10O3/c1-3-5(4(2)7)6(8)9/h5H,3H2,1-2H3,(H,8,9)/p-1
The calculated root mean square relative deviation % (RMSD %) of the allyl acetoacetate + S-CO2, methyl acetoacetate + S-CO2, and ethyl acetoacetate + S-CO2 systems for five temperature sets ranges from (1.29% − 3.63%), (1.47% − 8.95%) and (2.17% − 3.87%), respectively.
The historic DHP nucleus was serendipito...
The current study is based on the facile synthesis of ethyl acetoacetate modified chitosan and surface-functionalized alginate composite beads (EAA-MCSA). Chemical modification of sodium alginate was carried out to graft amino thiocarbamate moiety (-OCSNHNH2) to entrench selectivity and specificity across the linear chains of preliminary polysaccharide.
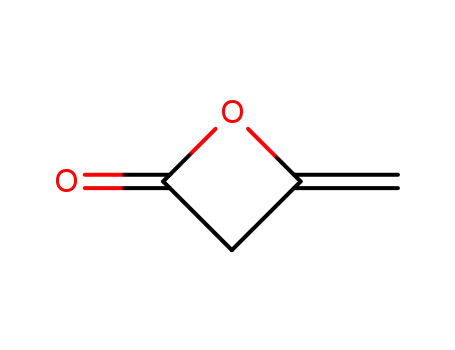
4-methyleneoxetan-2-one
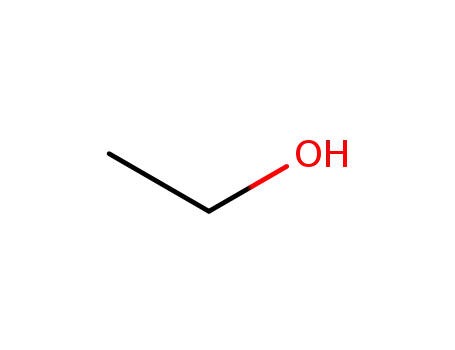
ethanol
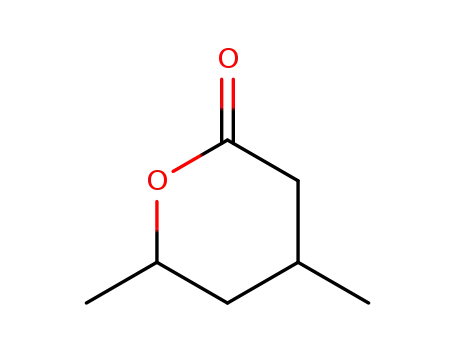
4,6-dimethyl-tetrahydro-pyran-2-one
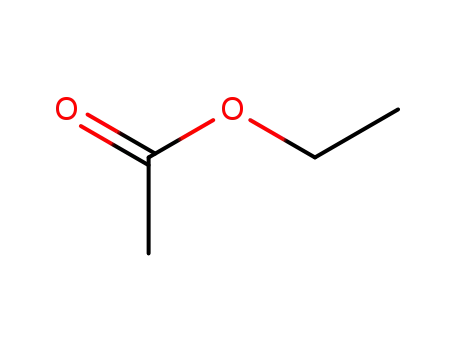
ethyl acetate
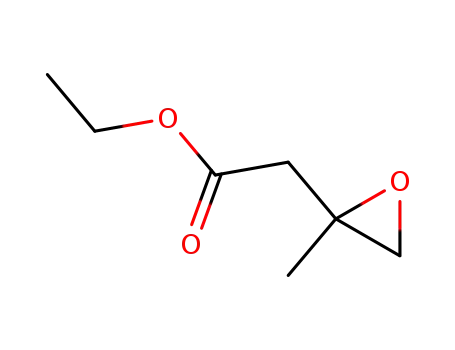
β,γ-epoxy-isovaleric acid ethyl ester
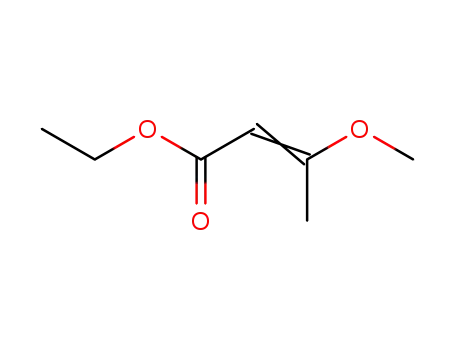
3-methoxy-but-2-enoic acid ethyl ester
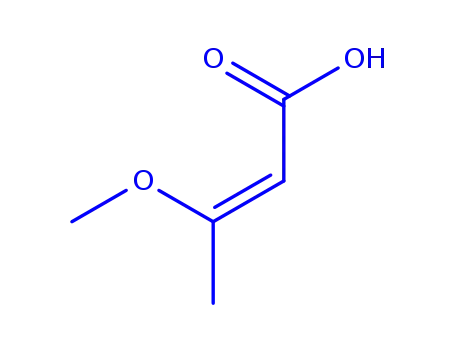
3-methoxy-2-butenoic acid
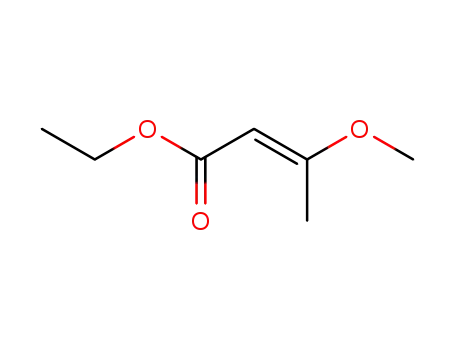
ethyl (2E)-3-methoxy-2-butenoate